
Introduction
Water is one of the most fascinating substances in the universe. It exists in three states—solid, liquid, and gas—under normal conditions. However, under specific conditions, water can simultaneously boil and freeze in what is known as the triple point. This remarkable phenomenon is a fundamental concept in thermodynamics and physics.
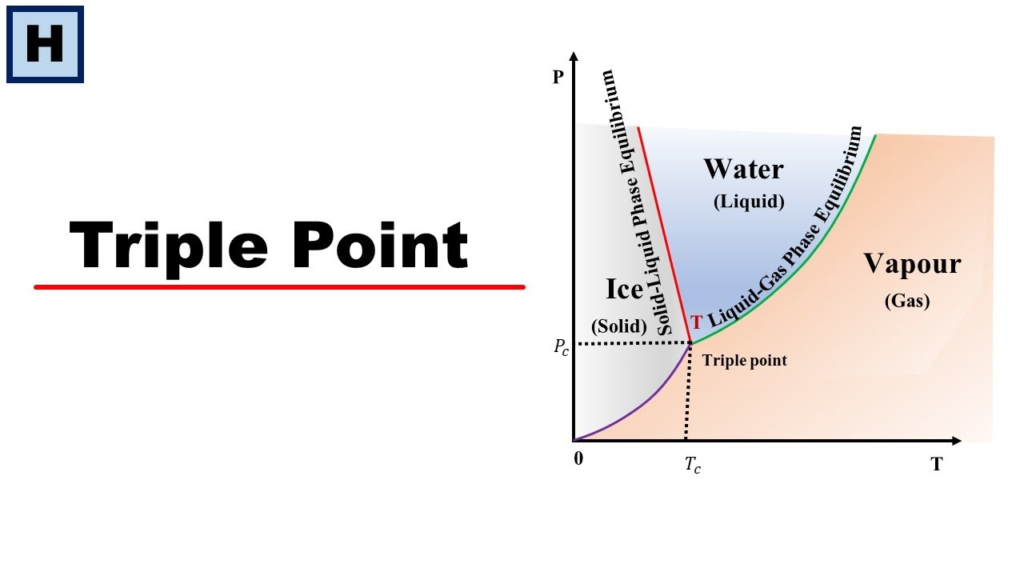
What is the Triple Point?
The triple point of water is the precise temperature and pressure at which it can exist in all three states—solid, liquid, and gas—simultaneously. For water, this occurs at 0.01°C (273.16 K) and 611.657 pascals (0.00604 atm) of pressure. Under these conditions, water molecules transition between phases without favoring any particular state, leading to the coexistence of ice, liquid water, and vapor.
How Does It Work?
To understand the triple point, consider the relationship between temperature, pressure, and phase transitions:
- Boiling occurs when a liquid turns into vapor. This happens when the vapor pressure of the liquid equals the external pressure.
- Freezing occurs when a liquid turns into a solid. This is influenced by temperature and pressure.
- At the triple point, both these conditions are met simultaneously. The system remains in a delicate equilibrium where some molecules freeze, some evaporate, and some remain liquid, all at the same time.

Experimental Demonstration
Scientists can create the triple point of water in a laboratory using a specially designed sealed chamber. By carefully adjusting the temperature and pressure inside the chamber, they can achieve the precise conditions required for the triple point. A commonly used demonstration involves a vacuum pump to lower the pressure of a small amount of water in a sealed tube, causing it to both boil and freeze at the same time.

Why Is the Triple Point Important?
- Thermodynamic Benchmark: The triple point of water is a fundamental reference point used in defining the Kelvin temperature scale.
- Meteorology and Climate Science: Understanding phase changes at different pressures helps in weather prediction and climate modeling.
- Space Exploration: The behavior of water in extreme conditions is critical for studying planetary environments where pressures and temperatures vary drastically.
- Industrial Applications: Cryogenics and chemical engineering use principles of phase transitions to optimize processes like refrigeration and material preservation.
Conclusion
The triple point of water is a stunning demonstration of the delicate balance of physics at the molecular level. It provides a deep insight into the principles of thermodynamics and phase transitions. While it may seem counterintuitive for water to boil and freeze at the same time, this phenomenon is a fundamental reality that plays a critical role in scientific research and technological advancements.


